Medical implants
Policy Briefing
Published 19/06/2019
Regulation of medical implants
Medical implants currently fall under EU regulation of medical devices, which is enforced by a competent authority in each member state. In the UK, this authority is the Medicines and Healthcare products Regulatory Agency (MHRA). At present, two EU Directives apply, which are implemented in the UK through the Medical Devices Regulations 2002.
Under these Directives, implants are classified as high-risk devices and their quality and safety must be independently certified before they can be sold in the EU. Certification is carried out by notified bodies, which are usually for-profit companies that are accredited by competent authorities.
Implants can be exempted from these requirements if they are manufactured and used to meet the needs of an individual patient or targeted patient groups.[iii] Implants can also be used ‘off-label’, for a purpose different to that for which they were certified. Where no treatment or device has worked or is available for a patient, implants that have not yet been certified can be used on compassionate grounds. Ethical issues raised by exceptional uses of implants are explored in the Nuffield Council on Bioethics briefing note on experimental treatments.
After the UK leaves the EU, it is likely that regulation of medical devices will remain aligned with EU regulation, though practical arrangements relating to certification and placing of devices on the market are likely to change.
Regulatory change
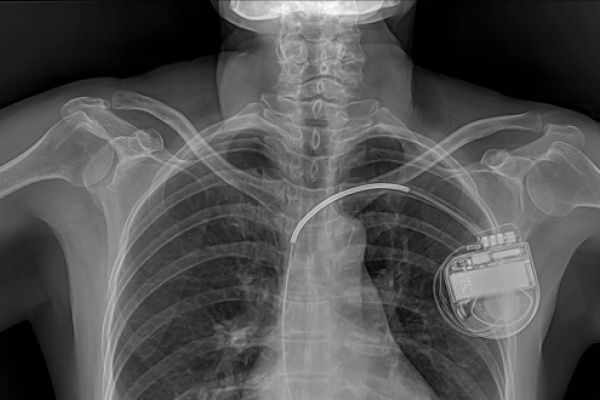
High profile incidents, such as the recall of a hip implant in 2010 which had failed in a large number of patients, triggered calls for regulatory change. Problems highlighted by critics included low requirements for the safety and efficacy of implants and insufficient oversight of notified bodies.
A new EU Medical Device Regulation was adopted in 2017, to be fully implemented by 2020, which aims to improve the safety of medical devices. It includes increased scrutiny of high-risk medical devices such as implants, and stricter criteria for the designation of notified bodies to ensure that they have the necessary expertise to evaluate evidence from manufacturers. The Regulation also aims to improve transparency around the evidence base for devices before and after they are approved. It requires that all medical implants should be registered on Eudamed, an EU-wide database, with a summary of performance and clinical data.
Some aspects of the new Regulation have been criticised. Though Eudamed is set to improve access to information about devices, there are calls for full clinical data to be made available to patients and clinicians. Commercial confidentiality is protected under the Regulation and full transparency about how implants have been approved, and about adverse events is not required. The for-profit nature of notified bodies has been highlighted as raising potential conflicts of interest as they are in competition with each other for applications from manufacturers. A more general criticism is that the Regulation is written in vague language, and therefore is open to very different interpretations.
Share