Medical implants
Policy Briefing
Published 19/06/2019
What are medical implants?
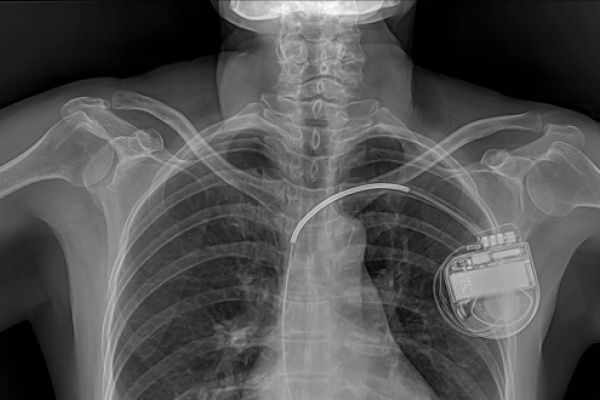
Medical implants are devices made from synthetic materials that are placed inside the human body for medical purposes, usually for long periods of time.* They can be used to replace body parts such as hips or knees, deliver medication such as for pain relief, monitor and regulate body functions such as heart rate, and provide support to organs and tissues. Some implants are inert and intended to provide structural support such as surgical meshes or stents. Others are active, in that they interact with the body, for example: by sending out electric shocks in response to changes in heart rhythm. Some implants are connected to systems outside the body (see Box 1).
Box 1. Connected implants
Implants that are connected, sometimes called ‘smart’ implants, communicate wirelessly with external devices. This type of implant includes pacemakers, implantable defibrillators, and neurostimulators, which monitor and automatically deliver treatment in response to changes in the body. They can store, collect, process, and transmit data about the patient and the implant, and receive instructions and software updates. Data might be transmitted from the implant when the patient is in hospital, or via the internet to allow remote control and monitoring of the patient.
Notes
Implants can also be used for cosmetic, lifestyle or personal enhancement purposes, and the line between these purposes can be blurred. For example, reconstructive breast implants following cancer treatment could be considered both medical and cosmetic, see Nuffield Council on Bioethics (2017) Cosmetic procedures: ethical issues.
Share