Policy briefing19th June 2019
Key points
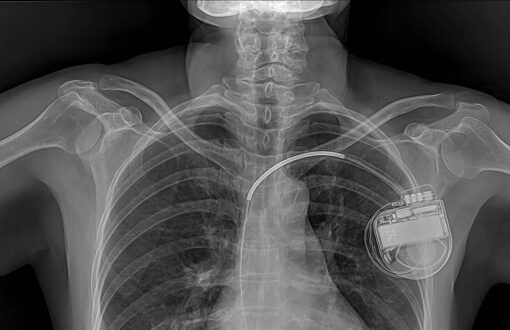
- Medical implants can be used to treat or monitor health conditions, or to restore body function.
- High-profile cases involving failing implants causing harm to patients have triggered a review of regulation to strengthen evidence and safety requirements for implants.
- Ethical issues arise in relation to equitable patient access to implants, the responsibilities of healthcare professionals in offering and monitoring medical implants, uncertainty about the long-term effects of implants and the problems this can pose for decision making, and liability when something goes wrong.
- Increasingly, implants are network-enabled, which expands possibilities for data gathering, monitoring, and analysis. This also might make implants more vulnerable to error and attack.
- Challenges for policy-makers include ensuring effective post-market surveillance of implants, promoting innovation that addresses patient need, and preparing for data and cybersecurity risks associated with connected implants.