Press Release
Medical implants raise safety and cybersecurity challenges for policy-makers
These challenges include ensuring effective post-market surveillance of implants to monitor their safety and efficacy, and preparing for data and cybersecurity risks associated with connected implants.
The medical implant market is thriving in the UK, with new devices emerging at a much higher rate than medicines. Rapid advances in the field are being driven by scientific developments in the field, and demand for implants is likely to increase due to an ageing population.
Safety and efficacy of medical implants
Some features of medical implants create challenges for assessing their efficacy and safety while ensuring timely access for patients. Because of the invasiveness of implants and their long-term presence in the body, conducting clinical research can be difficult. Also, the success of an implant can be dependent on a range of factors, such as the surgeon’s experience.
These challenges place particular responsibilities on manufacturers, regulatory bodies, and healthcare professionals to ensure that implants are used in a responsible and trustworthy manner, and are carefully monitored to ensure that any problems are discovered early.
There are calls for a UK-wide registry to track all new implants. The Government has commissioned an independent review of the safety of medical devices, in part triggered by concerns about vaginal mesh, and is considering establishing a national medical devices registry.1
Connected implants
Connected or ‘smart’ implants monitor and automatically deliver treatment in response to changes in the body, and connect wirelessly with external devices to transmit and receive information and instruction. Connected implants open up possibilities for improving patient care, but they can be vulnerable to error and attack, and raise privacy issues. Current regulations do not include requirements to demonstrate cyber security of implants before they can be approved.
Hugh Whittall, Director of the Nuffield Council on Bioethics, says:
“The medical implants market is thriving and it’s likely to continue to do so. Medical implants can greatly improve a person’s quality of life, even save their life, but their invasive nature leads to challenges in testing for safety and efficacy, and raises a number of ethical considerations. In light of recent cases of patients coming to significant harm through the use of medical implants, and the emergence of ‘smart’ implants, our briefing note aims to help guide policy-makers in promoting innovation in the sector to address patient need, while ensuring equitable and timely access to safe and effective implants.”
Notes to editors
Contact
Sarah Walker-Robson
Communications Manager
Tel: 020 7681 9619 / Mob: 07821 449 725
swalker-robson@nuffieldbioethics.org
References:
About the Nuffield Council on Bioethics
The Nuffield Council on Bioethics is an independent body that has been advising policy makers on ethical issues in bioscience and medicine for more than 25 years. We are funded by the Nuffield Foundation, the Medical Research Council, and Wellcome.
This is the sixth in a series of bioethics briefing notes published by the Nuffield Council on Bioethics.
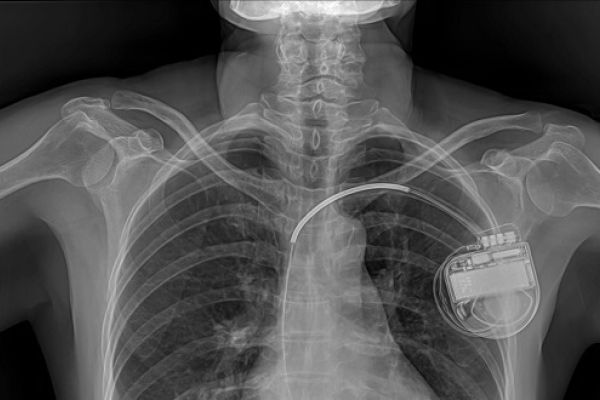
Medical implants
Read the briefing note
MEDICAL IMPLANTS
Share