10 May 2023
News
Techniques to prevent mitochondrial DNA disorders approved for use in clinics
The Human Fertilisation and Embryology Authority (HFEA) has permitted in principle the use of two different techniques - maternal spindle transfer (MST) and pronuclear transfer (PNT) - in clinics to prevent the transmission of mitochondrial DNA disorders. Clinics wishing to offer these procedures will now be able to apply to the HFEA for permission to do so. This will be the first time the techniques are offered in a fully regulated environment.
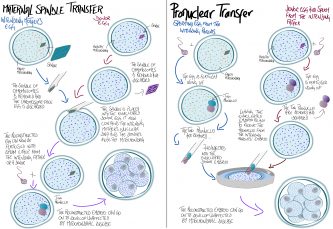
The Nuffield Council on Bioethics’ 2012 report concluded that the use of these techniques could be an ethical treatment option for couples at risk of having children with severe, inherited mitochondrial disorders, if they were shown to be acceptably safe and effective, and subject to prospective parents receiving appropriate information, counselling and support.
An independent expert panel convened by the HFEA to review the latest evidence of safety and efficacy for these techniques, concluded in November this year that it would be appropriate to offer mitochondrial donation techniques as a treatment for carefully selected patients to reduce the risk of a child being born with a mitochondrial DNA disorder.
The panel suggested a cautious adoption of MST and PNT, initially offering them only in cases where preimplantation genetic diagnosis (PGD) would be inappropriate because all embryos would be likely to be affected. In these cases, MST or PNT would offer a greater chance of success in preventing mitochondrial disease in their genetically related children. The panel also recommended that patients who become pregnant following MST or PNT should be offered prenatal testing.
Any clinics that become licensed to provide either MST or PNT will need to submit an application to the HFEA for each patient they wish to treat, which will be considered on a case-by-case basis. In their applications to the HFEA, clinics will need to provide evidence of the suitability of the patient according to selection criteria set out by the HFEA, and the competence of the embryologists who would carry out the procedure.
The Council’s report also recommended that the treatment should only be offered as part of a research study in centres specialising in mitochondrial disorders, and that centres should have arrangements for long term follow up of patients and children resulting from the treatment. These recommendations have been incorporated in the HFEA’s licensing guidance, which states that clinics will be required to have in place a documented process for monitoring children born following mitochondrial donation, where patients have given their consent to participating in follow-up. Clinics should also submit an annual report on patient uptake of follow-up studies and non-patient-specific information on the outcomes.
This decision only applies to the use of MST or PNT for the prevention of the transmission of mitochondrial disorders to offspring during reproduction. Clinical use of the techniques for other purposes, such as for infertility, remains illegal in the UK.Find out more about the Council's work on mitochondrial DNA disorders.

